Imagine the situation: you want to launch the production of a new line of dietary supplements, and you contact the local Sanepid for registration of the plant. In response, you hear that in order to register a plant, you must first report (notify) to the Main Sanitary Inspectorate a specific product that will be produced there. Sounds like bureaucratic absurdity? It is a classic conflict of competence, in which the entrepreneur becomes hostage to the lack of coherence between offices and falls into the regulatory trap of “paragraph 22”.
The world of dietary supplement regulation is full of nuances, and misconceptions about who is responsible for what — the contract manufacturer or the brand owner — can lead to costly mistakes, delays and financial penalties. This confusion is a problem that even experienced players in the market face.
In this article we will dispel the most common doubts. We will present 5 key, often surprising facts that clearly show what the division of duties looks like in the light of the law. After reading, you will know exactly who is really responsible for your supplement.

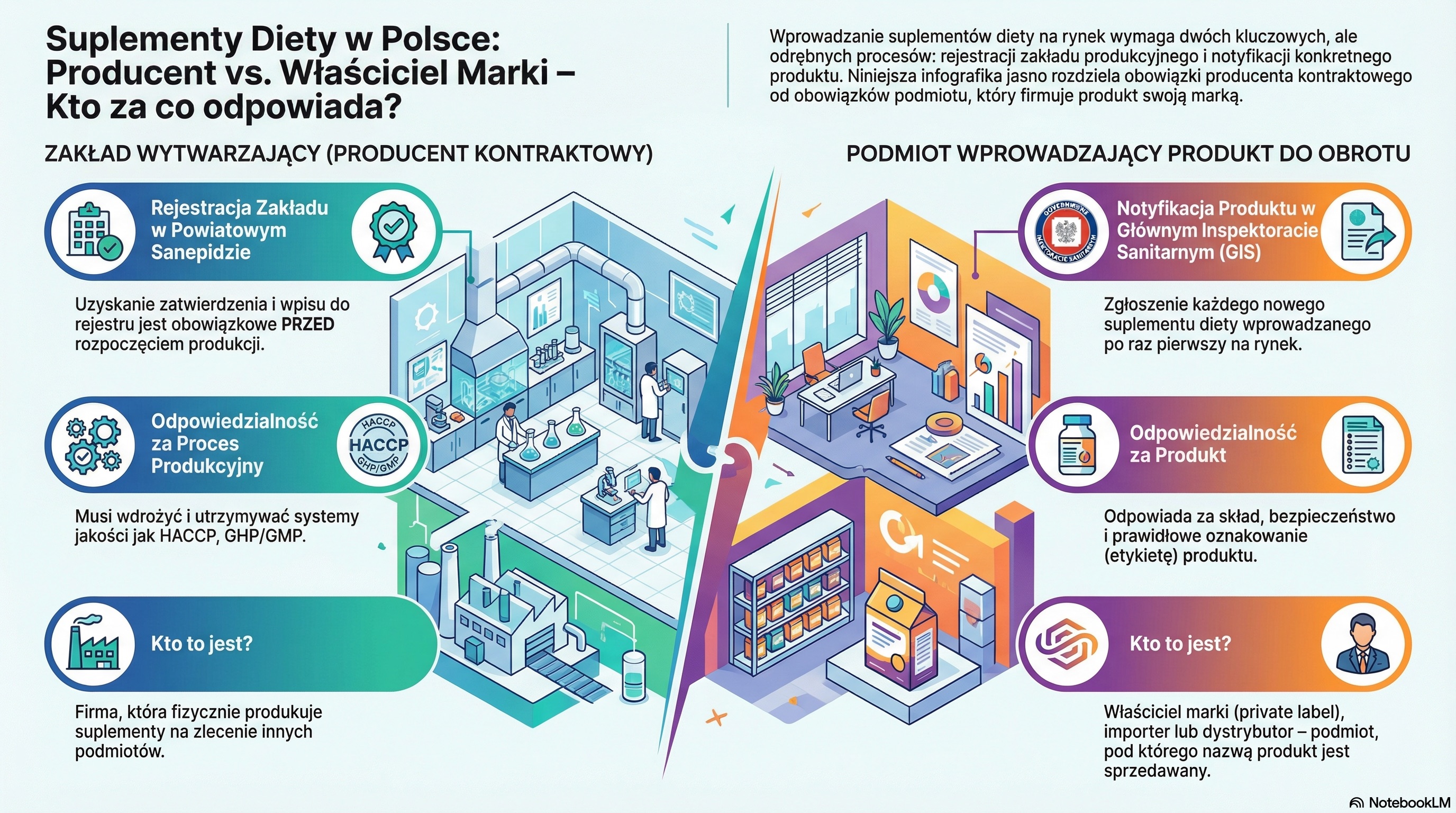
In the supplement industry, it is crucial to understand the fundamental difference between two entities whose roles are often confused.
These are two completely different roles, with separate legal obligations. Just because Company X has manufactured the capsules for you does not mean that they are responsible for what is written on their label and whether they have been properly reported to the office.
Contrary to intuition, and sometimes even against the erroneous suggestions of some officials, food law imposes a strict sequence of actions. It is not possible to declare a product if the place of its manufacture is not formally approved.
The correct process consists of two steps:
This rule is confirmed by the official position of the sanitary authorities. As we read on the website of the Voivodship Sanitary and Epidemiological Station in Warsaw:
Before applying for a GIS supplement, the registration or approval of the establishment must be made in accordance with Articles 61 and 63 of the Act.
It is worth remembering the key deadline: the application for registration of the plant must be submitted at least 14 days before the planned start of business.
The logic of this process is simple: the local Sanepid is responsible for the supervision of physical place production, guaranteeing its compliance with hygiene requirements. In turn, the central GIS manages the national registry Conceptual products placed on the market. First, there must be a legal place so that something can be legally produced and reported there.
Who should be on the label of your supplement? The manufacturer that physically manufactured it, or your company? The answer is unequivocal and follows directly from EU legislation.
According to Article 8 (1) Regulation (EU) No 1169/2011, the packaging must bear the particulars the entity under whose name or business the foodstuff is placed on the market.
In practice, it looks like this: if your company “Supplements A” outsources production to “Manufacturer B”, but sells the product under its own brand, then the label must contain the data of the company “Supplements A”. It is you, as the brand owner, who are fully responsible for all the information on the label - from the composition, to the nutritional statements, to the dosage.
Many entrepreneurs mistakenly believe that submitting a product to GIS is tantamount to its approval, verification or issuance of a “quality certificate” by the authority. This is a very popular and dangerous myth.
The procedure in GIS has the character Notifying, not licensing. This means that the General Sanitary Inspectorate only takes note of the fact that you are going to put the product in question on the market. GIS does not release any decision to place on the market. Verification of the legality of the product is usually carried out only at the stage of subsequent market control.
The entire responsibility for the compliance of the supplement with the law — its composition, safety and proper labeling — from start to finish lies with you as the marketing entity. However, the notification itself is mandatory, and the introduction of the product on the market without notification is fraught with a fine up to PLN 50,000and the risk of product withdrawal.
If the brand owner is responsible for the product, what exactly does the plant that produces it do? Its role is strictly defined and focused on ensuring safe and standards-compliant manufacturing conditions.
The main tasks of the contract manufacturer are:
In other words, the contract manufacturer is responsible for creating a legally functioning and safe environments for production. In turn, you, as the owner of the brand, are responsible for creating a legal product to the market.
As you can see, the regulations clearly separate the roles. The brand owner (responsible entity) is responsible for the product and its compliance with the law, and the contract manufacturer for the safe and hygienic process of its manufacture. Understanding this division is the absolute foundation for legal and safe operation in the dietary supplement market.
Therefore, the key tool to protect your business is a contract. Instead of asking yourself questions in the event of an inspection, make sure that your contract with the manufacturer precisely defines who is responsible for the product and what responsibilities each party has. Investing in clear legal arrangements at the very beginning is the best protection against costly misunderstandings and inspection problems in the future.
Checklists of health labels and claims — how not to waste weeks on corrections